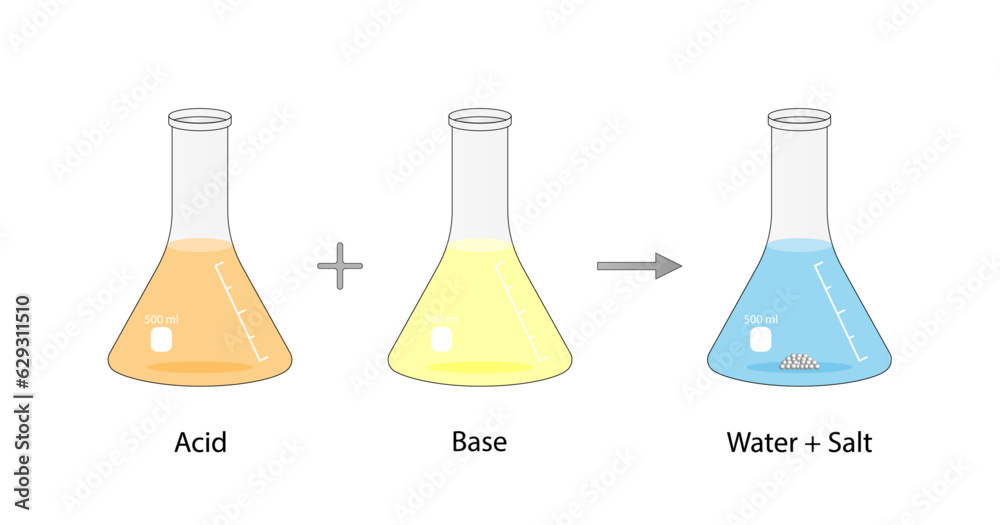
What Happens When You Mix Hcl And Naoh . Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. What happens when an acid such as hcl is mixed with a base. Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. All of the hcl reacts, and the amount of. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. In order to neutralize hcl, there must be equivalent. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web this video shows what happens when an acid and a base are made.
from fity.club
Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. All of the hcl reacts, and the amount of. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. What happens when an acid such as hcl is mixed with a base. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. Web this video shows what happens when an acid and a base are made. In order to neutralize hcl, there must be equivalent.
Neutralization Reaction Of Hcl And Naoh
What Happens When You Mix Hcl And Naoh Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. All of the hcl reacts, and the amount of. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. In order to neutralize hcl, there must be equivalent. Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web this video shows what happens when an acid and a base are made. What happens when an acid such as hcl is mixed with a base.
From brainly.in
calculate the ph of solution obtained by mixing equal volumes of N/10 What Happens When You Mix Hcl And Naoh All of the hcl reacts, and the amount of. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will. What Happens When You Mix Hcl And Naoh.
From www.numerade.com
SOLVEDexplain how a mixture of CH3COOH and CH3COONa+ in solution can What Happens When You Mix Hcl And Naoh Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. In order to neutralize hcl, there must be equivalent. All of the hcl reacts, and the amount of. Web this video shows what happens when an acid and a base are made. Web when the naoh and hcl solutions are mixed,. What Happens When You Mix Hcl And Naoh.
From www.pelajaran.guru
What Happens When You Mix Hcl And Naoh Buffer PELAJARAN What Happens When You Mix Hcl And Naoh Web this video shows what happens when an acid and a base are made. Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. Web in this video we determine the type of. What Happens When You Mix Hcl And Naoh.
From www.youtube.com
Following solutions were prepared by mixing different volumes of NaOH What Happens When You Mix Hcl And Naoh All of the hcl reacts, and the amount of. Web this video shows what happens when an acid and a base are made. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web in this video we determine the type of chemical reaction for the equation. What Happens When You Mix Hcl And Naoh.
From mungfali.com
HCl NaOH Titration What Happens When You Mix Hcl And Naoh Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. What happens when an acid such as hcl is mixed with a base. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. All of the hcl reacts,. What Happens When You Mix Hcl And Naoh.
From fity.club
Neutralization Reaction Of Hcl And Naoh What Happens When You Mix Hcl And Naoh Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. What happens when an acid such as hcl is mixed with a base. Web when the naoh and. What Happens When You Mix Hcl And Naoh.
From asideload7.gitlab.io
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With What Happens When You Mix Hcl And Naoh What happens when an acid such as hcl is mixed with a base. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. In order to neutralize hcl, there must be equivalent. Web this video shows what happens when an acid and a base are made. Web when. What Happens When You Mix Hcl And Naoh.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry What Happens When You Mix Hcl And Naoh All of the hcl reacts, and the amount of. In order to neutralize hcl, there must be equivalent. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl. What Happens When You Mix Hcl And Naoh.
From www.thoughtco.com
How to Prepare a Sodium Hydroxide or NaOH Solution What Happens When You Mix Hcl And Naoh In order to neutralize hcl, there must be equivalent. All of the hcl reacts, and the amount of. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web this video shows what happens when an acid and a base are made. Web when the naoh and. What Happens When You Mix Hcl And Naoh.
From fity.club
Naoh Reaction What Happens When You Mix Hcl And Naoh Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. Web this video shows what happens when an acid and a base are made. All of the hcl reacts, and. What Happens When You Mix Hcl And Naoh.
From www.pelajaran.guru
Reaksi Antara Naoh Dan Hcl Msds Sigma PELAJARAN What Happens When You Mix Hcl And Naoh Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. All of the hcl reacts, and the amount of. What happens when an acid such as hcl is mixed with. What Happens When You Mix Hcl And Naoh.
From fity.club
Acidbase Reaction Hcl Naoh Youtube What Happens When You Mix Hcl And Naoh In order to neutralize hcl, there must be equivalent. What happens when an acid such as hcl is mixed with a base. Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt. What Happens When You Mix Hcl And Naoh.
From www.chemicals.co.uk
The Science Behind Hydrochloric Acid The Chemistry Blog What Happens When You Mix Hcl And Naoh All of the hcl reacts, and the amount of. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web this video shows what happens when an acid and a base are made.. What Happens When You Mix Hcl And Naoh.
From slideplayer.com
Types of Solution Reactions ppt download What Happens When You Mix Hcl And Naoh Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. All of the hcl reacts, and the amount of. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate. Web when hydrochloric acid (hcl) and. What Happens When You Mix Hcl And Naoh.
From fity.club
Neutralization Reaction Of Hcl And Naoh What Happens When You Mix Hcl And Naoh Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. All of the hcl reacts, and the amount of. Web this video shows what happens when an acid and a base are made. Web a strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral. What Happens When You Mix Hcl And Naoh.
From www.numerade.com
SOLVED PART II HEAT OF NEUTRALIZATION OF STRONG ACID, HCL AND STRONG What Happens When You Mix Hcl And Naoh Web in this video we determine the type of chemical reaction for the equation naoh + hcl = nacl + h2o (sodium. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. Web this video shows what happens when an acid and a base are made. What happens. What Happens When You Mix Hcl And Naoh.
From go.isptutor.org
Chemical vs. Physical Reactions/Processes What Happens When You Mix Hcl And Naoh All of the hcl reacts, and the amount of. Web sodium hydroxide (naoh) mixed with hydrochloric acid (hcl), a simple acid base reaction, results in the formation of salt and water. Web when hydrochloric acid (hcl) and sodium hydroxide (naoh) are mixed together, they undergo a chemical reaction to form. In order to neutralize hcl, there must be equivalent. Web. What Happens When You Mix Hcl And Naoh.
From fity.club
Acidbase Reaction Hcl Naoh Youtube What Happens When You Mix Hcl And Naoh Web when the naoh and hcl solutions are mixed, the hcl is the limiting reagent in the reaction. Web this video shows what happens when an acid and a base are made. In order to neutralize hcl, there must be equivalent. What happens when an acid such as hcl is mixed with a base. Web sodium hydroxide (naoh) mixed with. What Happens When You Mix Hcl And Naoh.